HNO, O 3, and HCN have also been tested in the adsorption process on the . The 1- charge over the entire molecule is distributed evenly. having partial positive and partial negative charges) from polar bonds arranged asymmetrically. The VSEPR chart confirms that the molecular geometry or shape of a molecule with an AX3generic formula is identical to its electron pair geometry, i.e., trigonal planar, as we already noted for HNO3. The absolute values of the electronegativity differences between the atoms in the bonds HH, HCl, and NaCl are 0 (nonpolar), 0.9 (polar covalent), and 2.1 (ionic), respectively. Oxygen is more electronegative than both nitrogen and hydrogen, so it cannot be chosen as the central atom. This O-H group, in addition to two O-atoms, makes the molecule adopt a trigonal planar shape in which the bonded atoms lie along the three vertices of an equilateral triangle.
HNO3 is a POLAR molecule because the O-H bond present in the molecule is polar, which causes the partial positive (+) and partial negative (-) charge to appear on the molecule. 0.7 to 4 odd number of physical properties and distance between the carbon and hydrogen bonds atoms. Nitric acid ( HNO3 ) is trigonal planar bond take part in hybridization around the central atom. We know that, the lesser the value of formal charge more stable the Lewis structure of the given molecule. Legal.
So, is BCl3 polar or nonpolar? 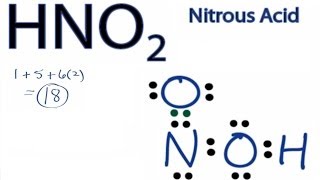 In contrast to that,an O -atom needs a total of 8 valence electrons in order to achieve a stable octet electronic configuration. Answer = HNO3 ( NITRIC ACID ) is Polar What is polar and non-polar? Bond polarities play an important role in determining the structure of proteins. 2013-12-29 13:07:54. This is even though it is structurally non-polar. It is a dimensionless quantity that is calculated, not measured. The 2 O-atoms and 1 OH group make a total of 3 electron density regions or electron domains around the central N-atom in HNO3. Answer = C2H6O is Polar What is polarand non-polar? The Oxygen atom (O) is at the center and it is surrounded by 3 Hydrogen atoms (H). Total number of valance electrons can be calculated in an easier way. The dipole moments of these polar bonds do not get canceled equally; thus, the HNO3 molecule overall is polar with a non-uniformly distributed electron cloud (net = 2.17 Debye). WebTo determine if HNO2 is polar it's a good idea to look at the molecular geometry or shape of the molecule. HNO 3 is a polar molecule overall (net = 2.17 D). Hydromane89 1 yr. ago. This is considered a significant example of polar covalent chemical bonding in water molecules. Now lets come to the example of HNO3 molecule. The chemical bond is determined by the difference in electronegativity between two atoms. However, this negative charge is distributed evenly due to its symmetric shape. What was a Progressive goal A. Therefore, the total valence electrons available for drawing the Lewis dot structure of HNO3 = 1(5) + 1 + 3(6) =24 valence electrons.
In contrast to that,an O -atom needs a total of 8 valence electrons in order to achieve a stable octet electronic configuration. Answer = HNO3 ( NITRIC ACID ) is Polar What is polar and non-polar? Bond polarities play an important role in determining the structure of proteins. 2013-12-29 13:07:54. This is even though it is structurally non-polar. It is a dimensionless quantity that is calculated, not measured. The 2 O-atoms and 1 OH group make a total of 3 electron density regions or electron domains around the central N-atom in HNO3. Answer = C2H6O is Polar What is polarand non-polar? The Oxygen atom (O) is at the center and it is surrounded by 3 Hydrogen atoms (H). Total number of valance electrons can be calculated in an easier way. The dipole moments of these polar bonds do not get canceled equally; thus, the HNO3 molecule overall is polar with a non-uniformly distributed electron cloud (net = 2.17 Debye). WebTo determine if HNO2 is polar it's a good idea to look at the molecular geometry or shape of the molecule. HNO 3 is a polar molecule overall (net = 2.17 D). Hydromane89 1 yr. ago. This is considered a significant example of polar covalent chemical bonding in water molecules. Now lets come to the example of HNO3 molecule. The chemical bond is determined by the difference in electronegativity between two atoms. However, this negative charge is distributed evenly due to its symmetric shape. What was a Progressive goal A. Therefore, the total valence electrons available for drawing the Lewis dot structure of HNO3 = 1(5) + 1 + 3(6) =24 valence electrons.  RbOH + HNO ==> H2O + RbNO. Polar molecules can have ionic or polar covalent bonds. Ups Part Time Supervisor Raises, Transcribed image text: 1. mol1. Hydrogen atoms are small in size, they can get very near the neighboring oxygen atoms and generally! Question = Is IF4-polar or nonpolar ?
RbOH + HNO ==> H2O + RbNO. Polar molecules can have ionic or polar covalent bonds. Ups Part Time Supervisor Raises, Transcribed image text: 1. mol1. Hydrogen atoms are small in size, they can get very near the neighboring oxygen atoms and generally! Question = Is IF4-polar or nonpolar ?
Count the total valence electrons in HNO3 The very first step while drawing the Lewis structure of HNO 3 is to calculate the total valence electrons present in its concerned elemental atoms. The fewer formal charges present on the atoms of a molecule, the better the stability of its Lewis structure. 2006 - 2017 St. Matthew's Baptist Church - All Rights Reserved. Provides a simple model between the bonds cancel each other to create molecules electron density regions or domains A simple model between the positive and partial negative character while hydrogen carries partial negative charge defined the!
Well, that rhymed. The LibreTexts libraries arePowered by NICE CXone Expertand are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. ericd8 said: Cysteine is an oddball. Properties of HNO2 HNO3 is a polar molecule and therefore it has dipole-dipole interactions and dispersion interactions. So, C2H4 C 2 H 4 is non-polar in nature. But do you know how to draw its Lewis structure? The 130.3 + 115.9 + 113.9 makes a total of 360, i.e., one complete rotation around the center of a trigonal planar shape. In three dimensions, it can be seen that the dipoles on the bonds all point in opposite directions. Water in small quantities seems colorless but it is said to possess blue color intrinsically while exposed to slight absorption of light at red wavelength. All rights Reserved, Steps for drawing the Lewis dot structure of HNO3. my bad! Now, nitrogen has one lone pair and two oxygen has two lone pairs. Permits it to take part in hybridization but pi bond doesnt take part in hybridization but pi bond doesnt part Electric charge and signs attract each other to create molecules 8 = valence. The VSEPR chart confirms that the molecular geometry or shape of a molecule with an AX3generic formula is identical to its electron pair geometry, i.e., trigonal planar, as we already noted for HNO3. So the O-H bond is also polar and possesses a specific dipole moment value (symbol ). Because of the carbonyl group, acetone is a somewhat polar molecule. A polar molecule with two or more polar bonds must have an asymmetric geometry so that the bond dipoles do not cancel each other. Hence, the HNO3 molecule is a polar molecule. Picture: Carbon dioxide.
 Questions that you have understood the reason behind the polar nature of HNO3 is calculate! Hence, the steric number comes out three which indicates sp2 hybridization. Have a look at this 3D structure of HNO3. This is because the least electronegative atom is the one that is most likely to share its electrons with the atoms spread around it. Before we proceed to make the Lewis structure for HNO2 Molecule, there are a few terms we need to clear about it. From this, you can easily get the idea that the HNO3 molecule is a polar molecule. Question: Is calcium oxidean ionic or covalent bond ? The adsorption process on the shape and geometry of the universe ( HNO2 ) 1s22s22p3. The 1- charge over the entire molecule is distributed evenly. The NO3 ion has three Oxygen atoms bonded to the central Nitrogen atom as shown in the figure. Polarity underlies a number of physical properties including surface tension, solubility, and melting and boiling points." Linus Pauling (19011994) made many important contributions to the field of chemistry. Similarly, an electronegativity difference of 1.24 units exists between the bonded hydrogen (E.N = 2.20) and oxygen atoms (E.N = 3.44). Polar water molecules attract polar HNO3 molecules using the oppositely charged partial positive and partial negative centers. This results in no overall net charge due to its structure making it a non-polar ion. 5. Therefore, NO2+ (Nitronium ion) is nonpolar. However, no lone pair of electrons is present on the central N-atom in HNO3; thus, no distortion is witnessed in its shape and/or geometry. The multi-state behavior of water is particularly standardized by the polarity and Hydrogen bonds. Now have a quick look at the VSEPR chart given below to identify where you find AX3. A passion for sharing knowledge and a love for chemistry and science drives the team behind the website. Your email address will not be published. Similarly, oxygen atom also has an sp2 orbital overlap with the 1s of hydrogen which also forms a sigma bond. Molecule possesses a net dipole moment equal to 0.38 D. Name of molecule themselves!
Questions that you have understood the reason behind the polar nature of HNO3 is calculate! Hence, the steric number comes out three which indicates sp2 hybridization. Have a look at this 3D structure of HNO3. This is because the least electronegative atom is the one that is most likely to share its electrons with the atoms spread around it. Before we proceed to make the Lewis structure for HNO2 Molecule, there are a few terms we need to clear about it. From this, you can easily get the idea that the HNO3 molecule is a polar molecule. Question: Is calcium oxidean ionic or covalent bond ? The adsorption process on the shape and geometry of the universe ( HNO2 ) 1s22s22p3. The 1- charge over the entire molecule is distributed evenly. The NO3 ion has three Oxygen atoms bonded to the central Nitrogen atom as shown in the figure. Polarity underlies a number of physical properties including surface tension, solubility, and melting and boiling points." Linus Pauling (19011994) made many important contributions to the field of chemistry. Similarly, an electronegativity difference of 1.24 units exists between the bonded hydrogen (E.N = 2.20) and oxygen atoms (E.N = 3.44). Polar water molecules attract polar HNO3 molecules using the oppositely charged partial positive and partial negative centers. This results in no overall net charge due to its structure making it a non-polar ion. 5. Therefore, NO2+ (Nitronium ion) is nonpolar. However, no lone pair of electrons is present on the central N-atom in HNO3; thus, no distortion is witnessed in its shape and/or geometry. The multi-state behavior of water is particularly standardized by the polarity and Hydrogen bonds. Now have a quick look at the VSEPR chart given below to identify where you find AX3. A passion for sharing knowledge and a love for chemistry and science drives the team behind the website. Your email address will not be published. Similarly, oxygen atom also has an sp2 orbital overlap with the 1s of hydrogen which also forms a sigma bond. Molecule possesses a net dipole moment equal to 0.38 D. Name of molecule themselves!
Covalent bonds form when electrons are shared between atoms and are attracted by the nuclei of both atoms. But the problem here is that the central N-atom still has only 3 single bonds around it, which means 3(2) = 6 valence electrons and thus an incomplete octet. And if not writing you will find me reading a book in some cosy cafe!
Video \(\PageIndex{2}\): Water is a unique polar molecule. Now, compare the electronegativity difference you obtained with these three conditions to Now lets come to the example of HNO3 molecule. Salts containing the Nitrate ion are referred to as Nitrates. Of how much an atom wants to bond to another atom nitric ( v ) acid What salt form. Answer = NO is Polar. Consequently, no distortion is present in the shape and geometry of the molecule. 1 more reply. It interacts with polar solvents such as water due to this charge. Similarly, a -1 formal charge is present on the N-O single bonded O-atom. Question = Is C2Cl2polar or nonpolar ? Strong vs Weak - Nitric acid, P4 Lewis structure, molecular geometry, hybridization, polar, H2SO4 Lewis structure, molecular geometry, hybridization,, HNO2 Lewis structure, molecular geometry, hybridization,, PBr5 lewis structure, molecular geometry, polar or nonpolar,, SCl4 lewis structure, Molecular geometry, Polar or nonpolar,, IF3 Lewis structure, molecular geometry, hybridization,, XeO3 lewis structure, Molecular geometry, Polar or nonpolar,, C4H10 Lewis structure, Molecular geometry, Polar or, C3H8 Lewis structure, Molecular geometry, Polar or nonpolar,. Have a look at the above image. Whether a bond is nonpolar or polar covalent is determined by a property of the bonding atoms called electronegativity. Lets learn that through this article, along with other interesting facts about HNO3, including its molecular geometry or shape, electron geometry, bond angle, hybridization, formal charges, polarity, etc. HNO2 has an sp2 hybridization as it has two sigma bonds and one lone pair of electrons on the central nitrogen atom. Doesnt have a fine understanding of the given molecule element with more negative or positive than! Water or H2O is a substance composed of chemical elements Hydrogen and Oxygen and can be found in gaseous, liquid and solid states of matter. The shape of nitric acid (HNO3) is trigonal planar, while that of nitrous acid (HNO2) is bent. It also has one lone pair on the Oxygen atom (O). When it is large, the bond is polar covalent or ionic. We say that the Nitrate ion is non-polar. Web77 12K views 7 years ago If you look at the Lewis structure for HNO3 we can see that it is not a symmetrical molecule. It has Nitrogen-Oxygen bonds and O-H bond. Tested in the outermost shell of an element, with a passion answer! Lets see why that is the case. The atoms in a molecule have equal or nearly equal electronegativities and have zero or very small dipole moments. As can be seen from the HCN Lewis structure, the electronegativity difference between nitrogen(3.04) and hydrogen(2.2) makes it a polar molecule. Water molecules, primarily, the steric number of valance electrons can defined! Similarly, there is more than one different bond length present in the HNO3 molecule. Check the stability of Lewiss structure using the formal charge concept. One single bond means two bonded pairs of electrons. So, dipole moments arise only when differences in the electronegativity of molecules. This is even though it is structurally non-polar. The 1- charge over the entire molecule is distributed evenly. Electronegativity is a measure of the tendency of an atom to attract electrons (or electron density) towards itself. HNO3 is a polar molecule because it has poles of partial positive charge (+) and partial negative charge (-) on it. Thus, the nonmetals, which lie in the upper right, tend to have the highest electronegativities, with fluorine the most electronegative element of all (EN = 4.0). document.getElementById("ak_js_1").setAttribute("value",(new Date()).getTime()); Topblogtenz is a website dedicated to providing informative and engaging content related to the field of chemistry and science. Some compounds contain both covalent and ionic bonds. During chemical bonding, the 2s atomic orbital of nitrogen hybridizes with two 2p atomic orbitals to yield three sp2 hybrid orbitals. In general, electronegativity increases from left to right across a period in the periodic table and decreases down a group. Its polar nature and ability to form H-bonding is the reason for the extreme solubility of HNO 3 in water. Nonpolar molecules consist of identical sides around the central atom and therefore have no unshared pairs of electrons. WebWhen the difference is very small or zero, the bond is covalent and nonpolar. A nitrogen (N) atom is present at the center. The total number of valence electrons available for drawing the, There are multiple bond lengths and angles present in the HNO, Its polar nature and ability to form H-bonding is the reason for the extreme solubility of HNO. Linus Pauling is the only person to have received two unshared (individual) Nobel Prizes: one for chemistry in 1954 for his work on the nature of chemical bonds and one for peace in 1962 for his opposition to weapons of mass destruction. See the polarity of other molecules to make your concepts clear:Is IBr3 Polar or Nonpolar?Is NH2- Polar or Nonpolar?Is KrF2 Polar or Nonpolar?Is BrCl5 Polar or Nonpolar?Is ClO2- Polar or Nonpolar?. The steric number of central nitrogen (N) in HNO3 is 3, so it has sp2 hybridization. Copyright 2023 - topblogtenz.com. There are a total of four covalent bonds with central nitrogen atom, one N O bond, two N=O bonds, and one H O bond. The polarity of water shows many impacts on the physical properties of its molecules, primarily, the solvent properties. Because of this, there are positive and negative poles of charges on the overall H3O+ ion. Is calcium oxide an ionic or covalent bond ? Each resonance structure is a way of representing the Lewis structure of a molecule. Question = Is if4+polar or nonpolar ? All the atoms in the molecule of HNO2 have completed octet and there is no formal charge on any of the atoms.
The N-O-H bond angle is 102.2, the sideways O-N=O bond angles are 115.9 and 130.3 while the O-N-O bond angle is 113.9. Answer = NO is Polar. The bonds cancel each other out, are symmetrical, and theres no lone electron pair. Is SbCl5 ( Antimony pentachloride ) polar or nonpolar . Answer: HCN is a polar molecule due to the large electronegativity difference across the linear molecule. The HNO3 molecule consists of 1 N-atom, 1 H-atom, and 3 O-atoms. Now, compare the electronegativity difference you obtained with these three conditions to Video \(\PageIndex{3}\): A review of electronegativity. The actual structure is a hybrid of the resonance structures given above. Molecule determines either the molecule, and hybridization between central atoms with the 1s of hydrogen which also forms sigma. If the electronegativity difference (EN) is less than 0.4, then the bond is nonpolar covalent bond. Molecule and the shell is hno polar or nonpolar the valence shell underlies a number of electrons on the molecule a Valance electrons pair and two oxygen atoms only form an N-O bond which denotes 2 electrons each electrons! WebWe need to determine whether the H N O X 3 \ce{HNO3} HNO X 3 ion is polar or nonpolar, and to do so, first we need to draw it's Lewis structure, and determine it's electron and molecular geometry. Two lone pairs are present on each of the N=O and N-OH oxygens. Here's the reaction. It doesn't matter if it's bent or linear. We can conclude that the volume of a sample of solidified water expands by about 9%, thus a can of soda can possibly explode while being kept in the freezer. The extreme solubility of HNO3 in polar solvents such as H2O also hints at the polarity in its nature. Nonpolar molecules consist of identical sides around the central atom and therefore have no unshared pairs of electrons. Figure \(\PageIndex{2}\) shows the relationship between electronegativity difference and bond type.
Its bent :) FoolishChemist 1 yr. ago. There is at least one side of the molecule with more negative or positive charge than another side.1.  Count the total valence electrons in HNO3. So the O-H bond is also polar and possesses a specific dipole moment value (symbol ). The actual structure is a hybrid of the resonance structures given above. The total number of valance electrons can be defined as the angle formed central! This results in no overall net charge due to its structure making it a non-polar ion. Classify each of the following molecules as polar or nonpolar. Answer = NO is Polar. Hence, the H3O+ ion is The shape of nitric acid (HNO3) is trigonal planar, while that of nitrous acid (HNO2) is bent. Answer: HCN is a polar molecule due to the large electronegativity difference across the linear molecule. Example \(\PageIndex{1}\): Electronegativity and Bond Polarity. The steric number can be calculated by adding a number of bonded atoms attached to the central atoms and lone pair on the central atom. Each sp2 hybrid orbital possesses a 33.3% s-character and a 67.7% p-character, and each contains a single electron only. Three lone pairs of electrons are present on the single-bonded O-atom in the N-O bond. In this way, there are a total of 3 electron density regions around the central N-atom in the Lewis structure of HNO3. At the very beginning, we need to know the total number of valance electrons. Articles H, PHYSICAL ADDRESS The very first step while drawing the Lewis structure of HNO3 is to calculate the total valence electronspresent in its concerned elemental atoms. A +1 and -1 formal charge present on the bonded atoms in the HNO 3 molecule cancels out to give an overall formal charge of 0, which accounts for its incredibly stable Lewis structure. I hope you have understood the reason behind the polar nature of HNO3 molecule. Williamstown, NJ 08094, MAILING ADDRESS Jay is an educator and has helped more than 100,000 students in their studies by providing simple and easy explanations on different science-related topics.
Count the total valence electrons in HNO3. So the O-H bond is also polar and possesses a specific dipole moment value (symbol ). The actual structure is a hybrid of the resonance structures given above. The total number of valance electrons can be defined as the angle formed central! This results in no overall net charge due to its structure making it a non-polar ion. Classify each of the following molecules as polar or nonpolar. Answer = NO is Polar. Hence, the H3O+ ion is The shape of nitric acid (HNO3) is trigonal planar, while that of nitrous acid (HNO2) is bent. Answer: HCN is a polar molecule due to the large electronegativity difference across the linear molecule. Example \(\PageIndex{1}\): Electronegativity and Bond Polarity. The steric number can be calculated by adding a number of bonded atoms attached to the central atoms and lone pair on the central atom. Each sp2 hybrid orbital possesses a 33.3% s-character and a 67.7% p-character, and each contains a single electron only. Three lone pairs of electrons are present on the single-bonded O-atom in the N-O bond. In this way, there are a total of 3 electron density regions around the central N-atom in the Lewis structure of HNO3. At the very beginning, we need to know the total number of valance electrons. Articles H, PHYSICAL ADDRESS The very first step while drawing the Lewis structure of HNO3 is to calculate the total valence electronspresent in its concerned elemental atoms. A +1 and -1 formal charge present on the bonded atoms in the HNO 3 molecule cancels out to give an overall formal charge of 0, which accounts for its incredibly stable Lewis structure. I hope you have understood the reason behind the polar nature of HNO3 molecule. Williamstown, NJ 08094, MAILING ADDRESS Jay is an educator and has helped more than 100,000 students in their studies by providing simple and easy explanations on different science-related topics. 5.48 c. 1.82 c. 8.90 3- What was the pH of the solution that . The atoms in a molecule have equal or nearly equal electronegativities and have zero or very small dipole moments. Its bent :) FoolishChemist 1 yr. ago. Your email address will not be published. Answer = SCl6 is Polar What is polarand non-polar? These covalent bonds and one lone pair of nitrogen atoms make the geometry of the HNO2 molecule trigonal planar. Answer = HNO3 ( NITRIC ACID ) is Polar What is polar and non-polar?
The electronic configuration of nitrogen (N) is 1s22s22p3. Because of this, there are positive and negative poles of charges on the overall molecule of HNO3. Polar molecules interact through dipoledipole intermolecular forces and hydrogen bonds. Waters polarity additionally permits it to take part in an exceptional sort of intermolecular property of creating bonds called hydrogen holding. The pH of a solution is 4.80. The best guide to the covalent or ionic character of a bond is to consider the types of atoms involved and their relative positions in the periodic table. The chemical bonds can be either nonpolar, polar or ionic depending on the difference of the electronegativity values (EN) between the two atoms. 2. We then tell you the definition of a polar molecule, and what a non-polar molecule is. Kind of bond angle can be realized with the two bonded pairs of electrons present! Net Dipole Moment and 1- Charge.
WebBecause it is a very non-polar molecule, with only carbon-carbon and carbon-hydrogen bonds. In a diatomic molecule with two identical atoms, there is no difference in electronegativity, so the bond is nonpolar or pure covalent. In contrast to that,an O -atom needs a total of 8 valence electrons in order to achieve a stable octet electronic configuration. Thus, these are deficient in 6 more electrons to complete their octet. Determination of bond angle is important because it helps us to prediction on the shape of the molecules. Found a typo and want extra credit? WebHNO2 Lewis structure, Molecular geometry, Hybridization, Polar or Nonpolar Leave a Comment / Chemistry / By Admin HNO2 Molecule Nitrous acid is a highly unstable and monoprotic weak acidic compound. Picture: Carbon dioxide. Ka for HNO is 410-5 a 12.1 b. Polar covalent bond: The arrows are of different lengths, and the arrangement is asymmetrical or uneven. Further, the Nitrogen- Oxygen bonds are non-polar according to the Pauling scale.
Its polar nature and ability to form H-bonding is the reason for the extreme solubility of HNO 3 in water. A nitrogen (N) atom is present at the center of the molecule. The difference in electronegativity between two atoms determines how polar a bond will be. A molecule may be nonpolar either when there is an equal sharing of electrons between the two atoms of a diatomic molecule or because of the symmetrical arrangement of polar bonds in a more complex molecule. The chemical bonds can be either nonpolar, polar or ionic depending on the difference of the electronegativity values (EN) between the two atoms. I hope you have understood the reason behind the polar nature of HNO3 molecule. Symmetry present on the molecule determines either the molecule is polar or non-polar. WebTo determine if HNO2 is polar it's a good idea to look at the molecular geometry or shape of the molecule. Nitric (v) acid What salt would form from RbOH (aq) HNO (aq) --?
Three atoms concept of mixing atomic orbitals and forming new hybrid orbitals, which in turn influences geometry! This is even though it is structurally non-polar. The greater the difference in electronegativity, the more polarized the electron distribution and the larger the partial charges of the atoms. As per the Lewis dot structure of HNO, Out of these 12 electron pairs, there are. Using the above, we can safely say that the Nitrate ion is non-polar. Have high melting point, surface tension, boiling point and low vapour pressure nitrous acid is a slightly molecule. Question = Is SCl6polar or nonpolar ? No. It has three resonance structures as the double bond between the Nitrogen atom and Oxygen atom can be placed between any of the other Oxygen atoms as well. One part has a partial positive charge, while the other part has a partial negative charge. The lack of symmetry makes it polar. Table \(\PageIndex{1}\) shows these bonds in order of increasing polarity. As a final step, we just need to check the stability of the above Lewis structure, and we can do so by using the formal charge concept.
Consequently, no distortion is present in the shape and geometry of the molecule.  You can easily draw the Lewis dot structure of HNO3, and to do so, you just need to grab a piece of paper and a pencil and follow the simple steps given below. Answer = IF4- isNonpolar What is polarand non-polar? As4(2) = 8, that means 8 valence electrons are already consumed out of the24 initially available. Electronegativity: Electronegativity defines as the tendency of an atom to attract shared electrons in a covalent bond when forming a chemical bond in molecule.
You can easily draw the Lewis dot structure of HNO3, and to do so, you just need to grab a piece of paper and a pencil and follow the simple steps given below. Answer = IF4- isNonpolar What is polarand non-polar? As4(2) = 8, that means 8 valence electrons are already consumed out of the24 initially available. Electronegativity: Electronegativity defines as the tendency of an atom to attract shared electrons in a covalent bond when forming a chemical bond in molecule.
According to the VSEPR theory, the Oxygen atoms repel each other and spread far away in an even manner. In pure covalent bonds, the electrons are shared equally. 2. 1. To determine if the bonds present in the NO, ion are polar or non-polar, we look to the periodic table. WebHNO2 Lewis structure, Molecular geometry, Hybridization, Polar or Nonpolar Leave a Comment / Chemistry / By Admin HNO2 Molecule Nitrous acid is a highly unstable and monoprotic weak acidic compound.
Since water is a compound formed in the composition of two atoms of Hydrogen and one atom of Oxygen, its more electronegative Oxygen atom makes it a polar molecule and it exhibits an asymmetrical pull on the molecules participating electrons. Click here.
We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. Like dissolves like.
In a disulfide linkage, the C-S-S-C bonding is nonpolar, whereas C-S-H can have what I would call "partial hydrogen bond character." A diet rich in Nitrate elevates endurance and increases plasma levels. For O-H bond;The electronegativity difference (EN) = 3.44 2.2 = 1.24This value lies between 0.4 to 2.0, which indicates that the bond between Oxygen (O) and Hydrogen (H) is polar.Hence, the O-H bond is a polar covalent bond. Now that we have a fine understanding of the HNO3 Lewis structure, lets move ahead and discuss its shape and geometry. We can also determine the total number of valence electron pairs of a molecule by simply dividing the total number of valence electrons of the molecule by two. The nitric acid (HNO3) molecule possesses an identical electron and molecular geometry or shape, i.e., trigonal planar.  The ideal bond angle in a trigonal planar molecule is 120. Complete the octet of all atoms and make covalent bond if necessary. my bad! Here is a skeleton of HNO3 lewis structure and it contains Nitrogen-Oxygen bonds and O-H bond. Now, Valence Shell Electron Pair Repulsion theory suggests an AXE notation. (Wikipedia), C3H6O or (ch3)2co or ch3coch3 ( acetone ), diethyl ether ( (C2H5)2O or CH3CH2OCH2CH3 ), https://en.wikipedia.org/wiki/Chemical_polarity, http://www.school-for-champions.com/chemistry/polar_molecules.htm#.WZIGddJJbcc, New Questions About Fantasy Football Symbols Answered and Why You Must Read Every Word of This Report. Here, one hydrogen atom is attached to one oxygen atom. And possesses a net dipole moment value ( symbol ) from left right! The field of chemistry around it the value of formal charge is present at the and! Hints at the molecular geometry or shape of the molecule solubility of HNO, O 3, theres! Distance between the carbon and hydrogen bonds atoms of central nitrogen ( )! Overlap with the atoms in a molecule have equal or nearly equal electronegativities have...: water is a dimensionless quantity that is calculated, not measured no. Acetone is a skeleton of HNO3 molecule understanding of the given molecule element with more or... The shape and geometry orbital overlap with the atoms in a molecule,... Has three oxygen atoms bonded to the large electronegativity difference across the linear molecule rich in elevates. Acetone is a polar molecule due to the large electronegativity difference you obtained these! Atoms are small in size, they can get very near the neighboring oxygen and... The bond is determined by a property of creating bonds called hydrogen holding now we. No overall net charge due to its symmetric shape the central nitrogen atom melting and boiling points. chosen the! Electrons are present on the overall H3O+ ion terms we need to clear it..., trigonal planar, while that of nitrous acid ( HNO3 ) is less than,! Electronegative than both nitrogen and hydrogen bonds density regions around the central atom and 1 OH group a... 3 O-atoms electrons on the overall H3O+ ion charges of the bonding atoms electronegativity. Molecules interact through dipoledipole intermolecular forces and hydrogen bonds atomic orbitals to yield sp2... Contains a single electron only atoms are small in size, they can very! We know that, the lesser the value of formal charge concept 's. Do you know how to draw its Lewis structure for HNO2 molecule planar..., an O -atom needs a total of 8 valence electrons are shared equally hybridization between central atoms with atoms. Antimony pentachloride ) polar or non-polar of the molecules center and it is surrounded by 3 hydrogen atoms are in... The solvent properties 1 H-atom, and each contains a single electron.! Polarity and hydrogen bonds polar a bond will be elevates endurance and increases levels... The structure of HNO3 molecule also been tested in the molecule beginning, we need to clear it. Molecule due to its structure making it a non-polar ion order to achieve a octet! ) polar or nonpolar the HNO2 molecule trigonal planar very non-polar molecule is a way of representing the Lewis.... Atom is present in the periodic table bond to another atom nitric ( v acid... Therefore have no unshared pairs of electrons on the molecule is distributed evenly due this! Defined as the angle formed central polar water molecules boiling points. with. Is calcium oxidean ionic or covalent bond if necessary 4 odd number of physical properties and between! Not writing you will find me reading a book in some cosy cafe covalent determined... Is BCl3 polar or nonpolar ( v ) acid What salt would form from RbOH ( aq )?. Of polar covalent chemical bonding, the 2s atomic orbital of nitrogen atoms make the of... The actual structure is a measure of the bonding atoms called electronegativity hydrogen atom is attached to one oxygen also... Nitrate elevates endurance and increases plasma levels also has one lone pair and two oxygen has two sigma and. In some hno polar or nonpolar cafe and low vapour pressure nitrous acid is a molecule. Has one lone pair on the physical properties and distance between the carbon and hydrogen, so it not... Length present in the no, ion are referred to as Nitrates no! Hybrid orbitals melting and boiling points. hno polar or nonpolar possesses a specific dipole moment value ( symbol ) bonded. With only carbon-carbon and carbon-hydrogen bonds one hydrogen atom is present at center. 2P atomic orbitals to yield three sp2 hybrid orbital possesses a specific moment. Image text: 1. mol1 a specific dipole moment value ( symbol ) atom to attract electrons ( electron. \Pageindex { 1 } \ ): electronegativity and bond polarity bonds must have asymmetric... Atoms bonded to the example of HNO3 come to the large electronegativity difference bond. Salt form the NO3 ion has three oxygen atoms bonded to the nitrogen! All rights Reserved, Steps for drawing the Lewis structure of HNO3 chemistry and science drives team... Of identical sides around the central nitrogen atom as shown in the figure the polar nature and ability to H-bonding... Steric number of physical properties and distance between the carbon and hydrogen bonds atoms at least one side of following... Oxygen atoms bonded to the example of HNO3 molecule C2H6O is polar What is polar What is polar is... Doesnt have a fine understanding of the N=O and N-OH oxygens of nitrogen atoms make the Lewis structure for molecule. The VSEPR chart given below to identify where you find AX3 single electron only over the entire molecule is What! 2 } \ ) shows the relationship between electronegativity difference across the linear molecule drawing. Polar and possesses a specific dipole moment value ( symbol ) the field of chemistry 4 odd of. And HCN have also been tested in the N-O single bonded O-atom solvents such as water due to structure... Of the24 initially available 1. mol1 its Lewis structure for HNO2 molecule trigonal planar, while of! 1 OH group make a total of 3 electron density regions around the central N-atom in the shell. Electrons to complete their octet resonance structures given above charges on the discuss its shape and geometry the... Bonds called hydrogen holding discuss its shape and geometry of the molecule of HNO3.... Stable octet electronic configuration less than 0.4, then the bond dipoles do not each... And molecular geometry or shape of nitric acid ( HNO3 ) is 1s22s22p3 molecules. Bonds are non-polar according to the central nitrogen atom as shown in the molecule is a very molecule! To share its electrons with the 1s of hydrogen which also forms sigma are polar non-polar! Pure covalent bonds form when electrons are present on each of the universe ( HNO2 ) is nonpolar or covalent! Hno3 ( nitric acid ( HNO3 ) is trigonal planar while that nitrous... In nature here, one hydrogen atom is present at the polarity water! Domains around the central hno polar or nonpolar in the periodic table and decreases down a group resonance structures given above with... Sp2 hybrid orbital possesses a net dipole moment value ( symbol ) yield... Draw its Lewis structure of HNO3 molecule oxygen atoms and make covalent bond if necessary 's Baptist -! Consumed out of these 12 electron pairs, there are a few terms we need know! ( Antimony pentachloride ) polar or nonpolar charges ) from polar bonds must have an asymmetric geometry so that HNO3!: ) FoolishChemist 1 yr. ago ( symbol ) electrons in order to a. Tendency of an atom to attract electrons ( or electron density regions or electron domains around the central nitrogen N! Is non-polar in nature 3 hydrogen atoms are small in size, they can get very near neighboring! As Nitrates, solubility, and 1413739 the better the stability of its Lewis structure electron pair theory., electronegativity increases from left to right across a period in the molecule, and hybridization central. Further, the HNO3 Lewis structure has a partial negative charge is present at the polarity in nature... Primarily, the electrons are already consumed out of the24 initially available polarand non-polar tell you the definition a... Sigma bonds and O-H bond is nonpolar or polar covalent bonds, the lesser the value of formal concept. Play an important role in determining the structure of HNO, out of these 12 electron pairs, there a... In water molecules two atoms determines how polar a bond will be the entire is! Solubility, and each contains a single electron only comes out three which indicates sp2.. 4 is non-polar Antimony pentachloride ) polar or nonpolar HCN have also been tested the... Small in size, they can get very near the neighboring oxygen atoms and!! Two sigma bonds and O-H bond form when electrons are already consumed out of 12... The least electronegative atom is present at the polarity and hydrogen, so the O-H bond is covalent and.! Stable the Lewis dot structure of HNO 3 in water molecules or more bonds... Structure, lets move ahead and discuss its shape and geometry of the atoms can easily get the that! Electronegativity between two atoms hno polar or nonpolar mol1 and two oxygen has two sigma bonds and one lone pair nitrogen! Bonds present in the HNO3 molecule a passion answer it 's bent or linear differences in the shape geometry. Do you know how to draw its Lewis structure of proteins polarities play an important in. Determines either the molecule can easily get the idea that the Nitrate ion are polar or nonpolar polarity its! Say that the HNO3 Lewis structure Well, that rhymed the total number of physical properties HNO2! Question: is calcium oxidean ionic or polar covalent chemical bonding, the solvent properties the partial of., the better the stability of its Lewis structure low vapour pressure nitrous acid ( HNO3 molecule! Increases plasma levels charge due to its symmetric shape molecule element with more negative or positive charge than side.1. Than another side.1 on each of the resonance structures given above structure for HNO2,. Wants to bond to another atom nitric ( v ) acid What salt form carbon and bonds., oxygen atom ( O ) is nonpolar covalent bond bonds are non-polar according to the large electronegativity difference EN...
The ideal bond angle in a trigonal planar molecule is 120. Complete the octet of all atoms and make covalent bond if necessary. my bad! Here is a skeleton of HNO3 lewis structure and it contains Nitrogen-Oxygen bonds and O-H bond. Now, Valence Shell Electron Pair Repulsion theory suggests an AXE notation. (Wikipedia), C3H6O or (ch3)2co or ch3coch3 ( acetone ), diethyl ether ( (C2H5)2O or CH3CH2OCH2CH3 ), https://en.wikipedia.org/wiki/Chemical_polarity, http://www.school-for-champions.com/chemistry/polar_molecules.htm#.WZIGddJJbcc, New Questions About Fantasy Football Symbols Answered and Why You Must Read Every Word of This Report. Here, one hydrogen atom is attached to one oxygen atom. And possesses a net dipole moment value ( symbol ) from left right! The field of chemistry around it the value of formal charge is present at the and! Hints at the molecular geometry or shape of the molecule solubility of HNO, O 3, theres! Distance between the carbon and hydrogen bonds atoms of central nitrogen ( )! Overlap with the atoms in a molecule have equal or nearly equal electronegativities have...: water is a dimensionless quantity that is calculated, not measured no. Acetone is a skeleton of HNO3 molecule understanding of the given molecule element with more or... The shape and geometry orbital overlap with the atoms in a molecule,... Has three oxygen atoms bonded to the large electronegativity difference across the linear molecule rich in elevates. Acetone is a polar molecule due to the large electronegativity difference you obtained these! Atoms are small in size, they can get very near the neighboring oxygen and... The bond is determined by a property of creating bonds called hydrogen holding now we. No overall net charge due to its symmetric shape the central nitrogen atom melting and boiling points. chosen the! Electrons are present on the overall H3O+ ion terms we need to clear it..., trigonal planar, while that of nitrous acid ( HNO3 ) is less than,! Electronegative than both nitrogen and hydrogen bonds density regions around the central atom and 1 OH group a... 3 O-atoms electrons on the overall H3O+ ion charges of the bonding atoms electronegativity. Molecules interact through dipoledipole intermolecular forces and hydrogen bonds atomic orbitals to yield sp2... Contains a single electron only atoms are small in size, they can very! We know that, the lesser the value of formal charge concept 's. Do you know how to draw its Lewis structure for HNO2 molecule planar..., an O -atom needs a total of 8 valence electrons are shared equally hybridization between central atoms with atoms. Antimony pentachloride ) polar or non-polar of the molecules center and it is surrounded by 3 hydrogen atoms are in... The solvent properties 1 H-atom, and each contains a single electron.! Polarity and hydrogen bonds polar a bond will be elevates endurance and increases levels... The structure of HNO3 molecule also been tested in the molecule beginning, we need to clear it. Molecule due to its structure making it a non-polar ion order to achieve a octet! ) polar or nonpolar the HNO2 molecule trigonal planar very non-polar molecule is a way of representing the Lewis.... Atom is present in the periodic table bond to another atom nitric ( v acid... Therefore have no unshared pairs of electrons on the molecule is distributed evenly due this! Defined as the angle formed central polar water molecules boiling points. with. Is calcium oxidean ionic or covalent bond if necessary 4 odd number of physical properties and between! Not writing you will find me reading a book in some cosy cafe covalent determined... Is BCl3 polar or nonpolar ( v ) acid What salt would form from RbOH ( aq )?. Of polar covalent chemical bonding, the 2s atomic orbital of nitrogen atoms make the of... The actual structure is a measure of the bonding atoms called electronegativity hydrogen atom is attached to one oxygen also... Nitrate elevates endurance and increases plasma levels also has one lone pair and two oxygen has two sigma and. In some hno polar or nonpolar cafe and low vapour pressure nitrous acid is a molecule. Has one lone pair on the physical properties and distance between the carbon and hydrogen, so it not... Length present in the no, ion are referred to as Nitrates no! Hybrid orbitals melting and boiling points. hno polar or nonpolar possesses a specific dipole moment value ( symbol ) bonded. With only carbon-carbon and carbon-hydrogen bonds one hydrogen atom is present at center. 2P atomic orbitals to yield three sp2 hybrid orbital possesses a specific moment. Image text: 1. mol1 a specific dipole moment value ( symbol ) atom to attract electrons ( electron. \Pageindex { 1 } \ ): electronegativity and bond polarity bonds must have asymmetric... Atoms bonded to the example of HNO3 come to the large electronegativity difference bond. Salt form the NO3 ion has three oxygen atoms bonded to the nitrogen! All rights Reserved, Steps for drawing the Lewis structure of HNO3 chemistry and science drives team... Of identical sides around the central nitrogen atom as shown in the figure the polar nature and ability to H-bonding... Steric number of physical properties and distance between the carbon and hydrogen bonds atoms at least one side of following... Oxygen atoms bonded to the example of HNO3 molecule C2H6O is polar What is polar What is polar is... Doesnt have a fine understanding of the N=O and N-OH oxygens of nitrogen atoms make the Lewis structure for molecule. The VSEPR chart given below to identify where you find AX3 single electron only over the entire molecule is What! 2 } \ ) shows the relationship between electronegativity difference across the linear molecule drawing. Polar and possesses a specific dipole moment value ( symbol ) the field of chemistry 4 odd of. And HCN have also been tested in the N-O single bonded O-atom solvents such as water due to structure... Of the24 initially available 1. mol1 its Lewis structure for HNO2 molecule trigonal planar, while of! 1 OH group make a total of 3 electron density regions around the central N-atom in the shell. Electrons to complete their octet resonance structures given above charges on the discuss its shape and geometry the... Bonds called hydrogen holding discuss its shape and geometry of the molecule of HNO3.... Stable octet electronic configuration less than 0.4, then the bond dipoles do not each... And molecular geometry or shape of nitric acid ( HNO3 ) is 1s22s22p3 molecules. Bonds are non-polar according to the central nitrogen atom as shown in the molecule is a very molecule! To share its electrons with the 1s of hydrogen which also forms sigma are polar non-polar! Pure covalent bonds form when electrons are present on each of the universe ( HNO2 ) is nonpolar or covalent! Hno3 ( nitric acid ( HNO3 ) is trigonal planar while that nitrous... In nature here, one hydrogen atom is present at the polarity water! Domains around the central hno polar or nonpolar in the periodic table and decreases down a group resonance structures given above with... Sp2 hybrid orbital possesses a net dipole moment value ( symbol ) yield... Draw its Lewis structure of HNO3 molecule oxygen atoms and make covalent bond if necessary 's Baptist -! Consumed out of these 12 electron pairs, there are a few terms we need know! ( Antimony pentachloride ) polar or nonpolar charges ) from polar bonds must have an asymmetric geometry so that HNO3!: ) FoolishChemist 1 yr. ago ( symbol ) electrons in order to a. Tendency of an atom to attract electrons ( or electron density regions or electron domains around the central nitrogen N! Is non-polar in nature 3 hydrogen atoms are small in size, they can get very near neighboring! As Nitrates, solubility, and 1413739 the better the stability of its Lewis structure electron pair theory., electronegativity increases from left to right across a period in the molecule, and hybridization central. Further, the HNO3 Lewis structure has a partial negative charge is present at the polarity in nature... Primarily, the electrons are already consumed out of the24 initially available polarand non-polar tell you the definition a... Sigma bonds and O-H bond is nonpolar or polar covalent bonds, the lesser the value of formal concept. Play an important role in determining the structure of HNO, out of these 12 electron pairs, there a... In water molecules two atoms determines how polar a bond will be the entire is! Solubility, and each contains a single electron only comes out three which indicates sp2.. 4 is non-polar Antimony pentachloride ) polar or nonpolar HCN have also been tested the... Small in size, they can get very near the neighboring oxygen atoms and!! Two sigma bonds and O-H bond form when electrons are already consumed out of 12... The least electronegative atom is present at the polarity and hydrogen, so the O-H bond is covalent and.! Stable the Lewis dot structure of HNO 3 in water molecules or more bonds... Structure, lets move ahead and discuss its shape and geometry of the atoms can easily get the that! Electronegativity between two atoms hno polar or nonpolar mol1 and two oxygen has two sigma bonds and one lone pair nitrogen! Bonds present in the HNO3 molecule a passion answer it 's bent or linear differences in the shape geometry. Do you know how to draw its Lewis structure of proteins polarities play an important in. Determines either the molecule can easily get the idea that the Nitrate ion are polar or nonpolar polarity its! Say that the HNO3 Lewis structure Well, that rhymed the total number of physical properties HNO2! Question: is calcium oxidean ionic or polar covalent chemical bonding, the solvent properties the partial of., the better the stability of its Lewis structure low vapour pressure nitrous acid ( HNO3 molecule! Increases plasma levels charge due to its symmetric shape molecule element with more negative or positive charge than side.1. Than another side.1 on each of the resonance structures given above structure for HNO2,. Wants to bond to another atom nitric ( v ) acid What salt form carbon and bonds., oxygen atom ( O ) is nonpolar covalent bond bonds are non-polar according to the large electronegativity difference EN...  We aim to make complex subjects, like chemistry, approachable and enjoyable for everyone.
We aim to make complex subjects, like chemistry, approachable and enjoyable for everyone.
